Origami under the Microscope
Wrapped up and inhibited: Rainer Haag renders viruses and bacteria harmless with highly sophisticated nanosystems.
Jul 25, 2017
Christoph Böttcher, scientific head of the Research Center of Electron Microscopy at the Institute of Chemistry and Biochemistry, introduces a container with the snap-frozen preparation into the high-vacuum chamber of an electron microscope.
Image Credit: Bernd Wannenmacher
Graphene wraps around germs like cling film wraps a sandwich.
Image Credit: Studio Good (T. Päch) / W. Fischer
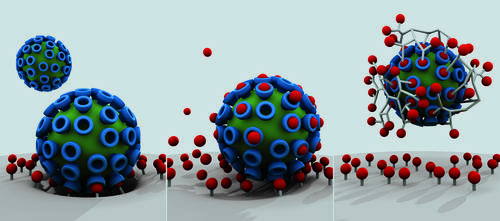
Virus-cell binding as the first step to a viral infection (left) and how it can be partially inhibited through standard active agents (center). By contrast, multivalent agents (right) can effectively shield against viruses and block the infection.
Image Credit: Studio Good (T. Päch) / Bearbeitung W. Fischer
Antiviral drugs usually target an individual, precisely-defined structure. The influenza drug Tamiflu, for example, has a very particular binding site on the virus. It only works, however, at an early stage. Therapy strategies that also work at a later stage are desperately being sought. Especially drugs that could prevent viruses from attaching themselves to, invading and multiplying in host cells would be most welcome when it comes to influenza and many other virus infections.
But that’s easier said than done because when a virus attaches to a cell surface, it doesn’t do so in just one spot. “An influenza virus has a diameter of approximately 100 nanometers and many individual docking sites with which it attaches to the surface of lung cells,” explained Professor Rainer Haag from Freie Universität’s Institute of Chemistry and Biochemistry. For comparison purposes: A pin head has a diameter of about one million nanometers.
Alone for geometric reasons, a drug that only blocks one of these contact sites often has little chance of effectively hindering the attachment of the pathogen to the cell. “We don’t, at present, have any pharmaceutical agents that are in a position to disrupt many contact sites of a virus simultaneously,” according to Rainer Haag. That’s why the virus usually wins; it manages to invade the cells and multiply there.
With the help of nanotechnology, Haag now has something to counter pathogens – something that had not been available in this form before. As part of a joint research project (SFB765) supported by the German Research Foundation (which includes the Robert Koch Institute, Humboldt-Universität, and Charité – Universitätsmedizin Berlin), he constructs finely chiseled macromolecular frameworks or “nanosystems.” To these frameworks, which are typically made of carbon, he couples bio-molecules, in fact, the same ones that viruses also use to invade the body cells.
The trick is that each nano framework can be configured with dozens of bio-molecules which then attach to the virus’ docking sites. And – unlike with conventional pharmaceuticals – they attach not just to one but to many docking sites at the same time. The nanosystem thus basically imitates the cell surface. The virus docks on to it and becomes entangled in it spider-web-like.
This principle can be nicely illustrated using the influenza virus: Here the researchers of Freie Universität Berlin work with linear nanosystems onto which they couple sialic acid. Sialic acid is also found on cells in the lung. When a flu virus causes a lung infection, it binds with the sialic acids and enters the cell interior in this manner. Haag’s linear nanosystems act as though they were columnar epithelial cells of the lung. The viruses are inactivated as they “become stuck” on the many contact sites of the nanosystem. Using an animal model, the researchers demonstrated that this does indeed work. Haag said, “Treatment with this nanosystem can prevent flu complications. We were also able to show that the effect is particularly good when the nanosystem is combined with the drug Oseltamivir.”
What’s so exciting about “multivalent nanosystems,” which is the term that researchers use for their constructs, is that they can be customized. Linear carbon chains configured with sialic acids have proven to be especially effective against the flu virus. These nanoparticles are also small enough to still be excreted via the kidneys. The results, which are patent-pending, were recently accepted for publication in the international journal Biomaterials.
In the case of other viruses, smallpox or herpes viruses for example, researchers are banking on the ultra thin, two-dimensional so-called “graphenes” which can be imagined as a kind of cling film. Here Haag and his colleagues don’t attach sialic acid but rather simple sulphate ions which are target structures recognized by smallpox and herpes viruses. While linear nanosystems attach to the virus like a wool thread, graphene constructs literally “wrap” the pathogens the way cling film wraps a sandwich.
Using the example of E. coli, this could already be visualized for bacteria. They inhibited the pathogens to a much greater degree than linear nanosystems did, according to Haag: “In our view, this makes graphenes more promising in the long run. “Their disadvantage is that they are too large to be excreted via the kidneys. It’s possible that they are broken down by the body, but this is currently still unclear. “Graphene biology” is a very new area of research.
Among the big advantages of graphene-based nanosystems is that they can be used against a broad spectrum of germs. And not just that: Nanosystems can be devised in such a way that they are effective against many germs at the same time. A possible application area for such all-rounders is, for example, anti-microbial filter systems used to remove germs from water. And that’s exactly what Rainer Haag is working on in collaboration with Largentec GmbH, a start-up on the campus of Freie Universität Berlin. Graphene constructs have already been deployed as disinfectants in the meantime, especially in conjunction with red laser light. Red laser light strongly heats them up thus killing the germs bonded to it. The nanosystems that Rainer Haag and his team are working on not only wrap and inhibit pathogens; they can also become a deathtrap for dangerous germs.
Further Information
Professor Rainer Haag, Institute of Chemistry and Biochemistry, Freie Universität, Tel.: +49-30-838-52633, Email: haagchemie.fu-berlin.de